February 4, 2010
3-D scaffold provides clean, biodegradable structure for stem cell growth
Medical researchers were shocked to discover that virtually all human embryonic stem cell lines being used in 2005 were contaminated. Animal byproducts used to line Petri dishes had left traces on the human cells. If those cells had been implanted in a human body they likely would have been rejected by the patient’s immune system.
Even today, with new stem cell lines approved for use in medical research, there remains a risk that these cells will be contaminated in the same way. Most research labs still use animal-based “feeder layers” because it remains the cheapest and most reliable way to get stem cells to multiply.
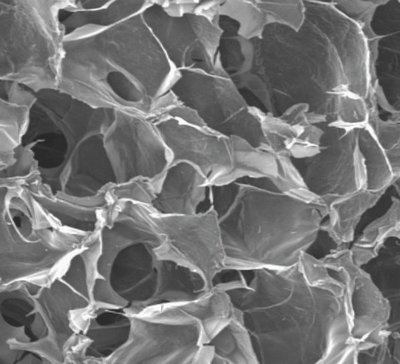
Materials scientists at the UW have now created an alternative. They built a three-dimensional scaffold out of a natural material that mimics the binding sites for stem cells, allowing the cells to reproduce on a clean, biodegradable structure. Results published in the journal Biomaterials show that human embryonic stem cells grow and multiply readily on the structure.
“The major challenge for stem cell therapy today is it’s very difficult to make a lot of them with high purity,” said lead author Miqin Zhang, a UW professor of materials science and engineering. “So far it seems like this material is very good for stem cell renewal.”
Medical researchers hope to someday use stem cells to grow new tissues and organs. Key to the research is the fact that new cells maintain the property that holds medical promise — the ability to differentiate into any of the more than 220 cell types in the adult human body.
Growing the cells in three dimensions better resembles conditions in the human body. It also allows mass production, which will be needed for any clinical applications.
“Three-dimensional scaffolds are an active area of research,” said Carol Ware, a UW professor of comparative medicine and expert on stem cells. “They are not commonly used yet, but will be important to transition embryonic stem cells to the clinic. To date, nobody has found a perfect matrix.”
Zhang’s cylindrical scaffold is made of chitosan, found in the shells of crustaceans, and alginate, a gelatinous substance found in algae. Chitosan and alginate have a structure similar to the matrix that surrounds cells in the body, to which cells can attach. Different processing techniques can make the scaffold out of interconnected pores of almost any size, Zhang said.
Researchers first seeded the scaffold with 500,000 embryonic stem cells, and after 21 days the scaffold was completely saturated. The cells infiltrated the structure, Zhang added, unlike other materials where cells often grow only on the surface.
“This scaffold mimics the extracellular matrix at the atomic level, and so the cells are able to grow in this environment,” Zhang said.
To retrieve the cells, researchers immersed the scaffold in a mild solution. The structure is biodegradable and so dissolved to release the stem cells. One also could implant the stem cell-covered scaffold directly into the body.
Analysis of gene activity and testing in the lab and in mice showed that the new stem cells retained the same properties as their predecessors.
Other researcher groups are also looking for alternatives to feeder layers. The leading contenders are scaffolds coated with custom proteins designed to mimic the key properties of the animal cells in the feeder layer. Such products are expensive and difficult to produce in a consistent manner, Zhang said. The proteins also get used up in a few days and have to be replaced, making them costly and time-consuming for everyday use.
“Our scaffold is made of natural materials that are already FDA-approved for food and biomedical applications. Also, these materials are unlimited, and the cost is cheap,” she said.
Zhang’s group is now working to build a scaffold larger than the current dime-sized prototype, and is collaborating with the UW’s Institute for Stem Cells and Regenerative Medicine and UW School of Medicine to try growing different types of stem cells, including those from umbilical cord blood and bone marrow, in the material. They will try to get the resulting cells to differentiate into bone, neuron, muscle and liver cells.
Co-authors are Zhensheng Li and Matthew Leung, UW doctoral students in materials science and engineering; Dr. Richard Hopper, an associate professor at the UW School of Medicine; and Dr. Richard Ellenbogen, professor and chair of neurological surgery at the UW School of Medicine.