Adding HSCT Studies
Download Alert: If you are trying to download the subaward form when using Chrome, you may get what appears to be an error page. See the steps for Chrome below for instructions.
Use the Add Studies section to add the appropriate studies to your Human Studies & Clinical Trails (HSCT) form.
You can add two types of studies:
- Full Study, which has five sections. There is also an option to import a full study.
- Delayed Onset Study, which has a single section.
Full Study
To add a full study, you can select Add New Full Study in the Add Studies section of the form to directly enter your data.
You also have the option of completing all or part of a PDF version of the full study, and then importing the PDF data into your Grant Runner application.
Use the Download Blank Study Form link to get a copy of the PDF form. Note: Other versions of this form will not upload properly.
Known issue: Steps for Chrome
When you click the “Download Blank Study Form” link, a page displays that refers to the version of Adobe Acrobat being used, and the file is not immediately downloaded. Look for the standard download icon on the upper, right corner of the page. Move your mouse to that area if the icon is not visible. Once you use the download icon, you will need to indicate where to save the file. Once saved, you can open the file and enter data.
Once you’re done filling in the form, select Import New Study to import your PDF. You can then edit the imported data, if needed.
You must include a Study Title (item 1.1) and answer all of the questions 1.4.a-1.4.d on your PDF before you can import it. If your opportunity does not allow clinical trials, or you have not answered “Yes” to all of the questions 1.4.a-1.4.d, then any data in your PDF for sections 4 and 5 will not be imported. For assistance in answering questions 1.4.a-1.4.d, review the NIH’s Definition of a Clinical Trial.
Navigating Full Studies
The full study part of the form is long, so we’ve given each section its own editable page in SAGE. As you work your way through completing the form, you can navigate back to any higher-level page by using the breadcrumb at the top of the page. At the bottom of each page is a button to return to the next higher-level page. Sections two and four also have subsections with the same navigation choices.
The sections and subsections for the Full Study are:
- Basic Information
- Study Population Characteristics
- Inclusion Enrollment Report subsection
- Protection and Monitoring Plan
- Protocol Synopsis
- Interventions subsection
- Outcomes and Measures subsection
- Other Clinical Trial Related Attachments
When you select Add New Full Study, the first page you will see is called “Full Study Details” as shown in the image below.
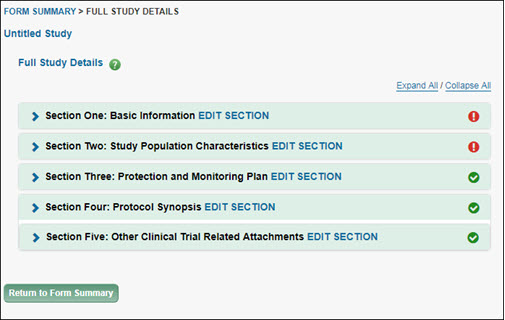
This page has several items to note:
- The breadcrumb, which on this summary page is “FORM SUMMARY > FULL STUDY DETAILS”. Use it to navigate through the parts of the study form.
- The study title, which is initially “Untitled Study” until you add your own title in Section One.
- The heading “Full Study Details” (with help icon).
- The list of the five sections that make up the Full Study. Each section has a box that will change color as you move your mouse across it. Each section box contains
- Its label, such as “Section One: Basic Information” with a “caret” to the left of the section label. Clicking on the caret expands the section in a read-only mode.
- An EDIT SECTION link to the right of the label; use it to open the section to add or edit data.
- An icon at the far right: a red exclamation point indicates required data is missing; a green check mark indicates that all required data has been added.
- Below the sections list is a button to “Return to Form Summary”. Selecting this re-displays the Form Summary page.
To enter data into a form section, select Edit Section to open the page for that section. Use the breadcrumb or the button at the bottom of each section page to navigate back to the Study Details page.
Section One: Basic Information
This section contains the following fields. Please refer to your funding opportunity announcement and agency-specific instructions for additional guidance.
| Field | Description |
|---|---|
| 1.1. Study Title | You must enter a unique title that describes the study. Your title will display at the top of the each page of the study. |
| 1.2. Is this Study Exempt from Federal Regulations?
1.3. Exemption Numbers |
You must answer Yes or No to this question.
If you answer Yes, you must indicate at least one Exemption Number. |
| 1.4. Clinical Trial Questionnaire | There are four questions. By default, question 1.4.a will be answered Yes and not be editable, since study records are only available when Human Subjects is Yes. Answer Yes or No as appropriate for the other 3 questions.
If the answers to all four questions are yes, then your study meets the definition of a Clinical Trial. For assistance in answering, review the NIH’s Definition of a Clinical Trial. The questions are:
|
| 1.5. Provide the ClinicalTrails.gov Identifier (e.g., NCT87654321) for this trial, if applicable | Enter your identifier. It starts with NCT and contains 8 digits. Once you have entered the full identifier, the button will be enabled. When you select the Import from ClinicalTrials.gov button, Grant Runner will check if the identifier is valid, and if so, will populate the HSCT Full Study with the following ClinicalTrials.gov fields:
The imported data will replace any previously entered data on the HSCT Full Study. After import, you can edit any of the information as necessary. |
Section Two: Study Population Characteristics
This section contains the following fields. Please refer to your funding opportunity announcement and agency-specific instructions for additional guidance.
| Field | Description |
|---|---|
| 2.1. Condition or Focus of Study | Select the Add New Condition link to enter the name(s) of the condition(s) you are studying or the focus of the study. See funding opportunity announcement and agency-specific instructions for additional guidance. |
| 2.2. Eligibility Criteria | List the study’s inclusion and exclusion criteria. |
| 2.3. Age Limits | Use these pairs of fields to enter the Minimum and Maximum Ages. Pick the relevant time unit from the drop-down menu. If data is entered for one of these field, it must be entered for both unless you select N/A (no limit) from the drop-down. |
| 2.3.a. Inclusion of Individuals Across the Lifespan | Add an attachment, if appropriate. |
| 2.4. Inclusion of Women and Minorities | Add an attachment, if appropriate. |
| 2.5. Recruitment and Retention Plan | Add an attachment, if appropriate. |
| 2.6. Recruitment Status | Select a value from the drop-down menu, if appropriate. Choices are:
|
| 2.7. Study Timeline | Add an attachment, if appropriate. |
| 2.8. Enrollment of First Participant | Enter an Enrollment Date, and select either Anticipated or Actual from the accompanying drop-down menu. If data is entered for one of these field, it must be entered for both. |
| 2.9 Inclusion Enrollment Report | Select the Add New Inclusion Enrollment Report link to open the subsection page. |
Section Three: Protection and Monitoring Plan
This section contains the following fields. Please refer to your funding opportunity announcement and agency-specific instructions for additional guidance.
| Field | Description |
|---|---|
| 3.1. Protection of Human Subjects | Add an attachment, if appropriate. |
| 3.2. Is this a multi-site study that will use the same protocol to conduct non-exempt human subjects research at more than one domestic site?
Single IRB plan |
Select Yes, No, or Not Applicable as appropriate. This question is not required.
Add an attachment, describing the Single IRB Plan, per your opportunity instructions |
| 3.3. Data and Safety Monitoring Plan | Add an attachment, if appropriate. |
| 3.4. Will a Data and Safety Monitoring Board be appointed for this study? | Select Yes or No, as appropriate. |
| 3.5. Overall Structure of the Study Team | Add an attachment, if appropriate. |
Section Four: Protocol Synopsis
This section contains the following fields. Please refer to your funding opportunity announcement and agency-specific instructions for additional guidance.
Note: If your opportunity does not allow clinical trials, then this section will not be editable. If your opportunity does allow clinical trials, this section will become editable when questions 1.4 a-d are all answered “Yes”.
| Field | Description |
|---|---|
| 4.1. Study Design | This item consists of sub items a through g, described in the following table rows. |
| 4.1.a Detailed Description | Enter the description of the protocol. |
| 4.1.b Primary Purpose | Select a value from the drop-down menu. Choices are:
|
| 4.1.c Interventions | Use the Add Intervention link to open a subsection. All three fields are required for each intervention you add. The fields are Intervention Type (choices listed below), Name, and Description. Use the breadcrumb or Return button to re-display Section Four.
Intervention Types are:
|
| 4.1.d Study Phase | Select a value from the drop-down menu. Choices are:
Also answer yes or no to the question “Is this an NIH-defined Phase III clinical trial?” |
| 4.1.e Intervention Model | Select a value from the drop-down menu. Choices are:
|
| 4.1.f Masking | Select Yes or No. If yes, you must also answer Yes or No to four masking types:
|
| 4.1.g Allocation | Select a value from the drop-down menu. Choices are:
|
| 4.2. Outcome Measures | Use the Add New Outcome or Measure link to open a subsection page. All four fields are required for each outcome or measure you add. The fields are:
Use the breadcrumb or Return button to re-display Section Four. |
| 4.3. Statistical Design and Power | Attach a PDF formatted file. |
| 4.4. Subject Participation Duration | Enter the time it will take for each individual participant to complete all study visits. See agency-specific instructions for additional guidance. |
| 4.5. Will the study use an FDA-regulated intervention?
4.5.a. If yes… |
Answer Yes or No.
If yes, attach a file that describes the availability of Investigational Product (IP) and Investigational New Drug (IND)/Investigational Device Exemption (IDE) status. |
| 4.6. Is this an application clinical trial under FDAAA? | Answer Yes or No. |
| 4.7. Dissemination Plan | Attach a PDF file. |
Section Five: Other Clinical Trial Related Attachments
This section has just one item: 5.1. Other Clinical Trial-related Attachments. Use the Add Attachment link to attach one or more files.
Note: If your opportunity does not allow clinical trials, then this section will not be editable. If your opportunity does allow clinical trials, this section will become editable once you have answered “Yes” to all of the questions 1.4 a-d.
Delayed Onset Study
To include a delayed onset study, select Add New Delayed Onset Study in the Add Studies section of the summary page. Delayed onset studies are those for which there is no well-defined plan for human subject involvement at the time of submission, per agency policies on Delayed Onset Studies.

You must provide a Study Title, answer Yes or No to the Anticipated Clinical Trial question, and add a Delayed Onset Study Justification attachment for the omission of human subjects study information.