May 1, 2001
Proteins are vastly more complicated than previously realized
For an animation of protein stretching, click here.
The function of proteins — the workhorses of our bodies — depends on how those proteins are physically folded. Researchers around the world are examining the countless complex structures of proteins and their functions to learn more about therapies for the human body. Protein folding has been compared in complexity to the folding of delicate origami.
While the folding process is already complicated, imagine trying to unfold a delicate origami crane back into a flat sheet of silk paper — while you’re in a wind tunnel. In fact, imagine trying to unfold the origami in a wind tunnel while countless other hands are also pulling at the paper. And yet, that’s comparable in complexity to what the hundreds of thousands of cells and proteins are doing in your body right now.
That’s because proteins and cells are locked together at numerous contact points. The movement of a cell stretches the proteins around it, and vice versa. A new University of Washington study says scientists are going to have to study how protein structures change when stretched before they understand how the body functions.
“The function of a protein is tightly controlled by its structure, yet there is very little information about how mechanical forces may change the structure of proteins,” says Dr. Viola Vogel, director of the University of Washington’s Center for Nanotechnology in the Department of Bioengineering. “Right now, it feels like we are only looking at part of the equation of how proteins work since we just know their equilibrium structures. If you do not know how mechanical forces change the function of cells and proteins, you will not understand different diseases that involve mechanical forces, such as hypertension.”
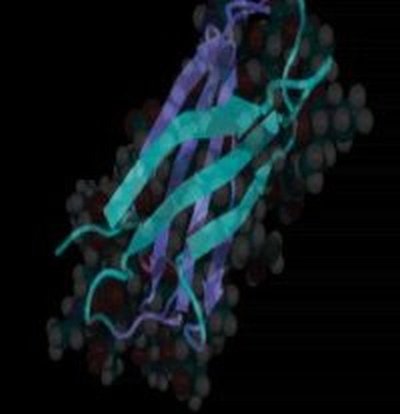
Vogel is one of the authors of “Comparison of the early stages of forced unfolding for fibronectin type III modules,” which appears in the May 1 Proceedings of the National Academy of Sciences, a journal of the National Academy of Sciences. The paper shows, at atomic resolution, how mechanical forces change the structure of a family of protein modules that fold into the same structures — yet have less than 20 percent of their amino acids in common.
Fibronectin is a useful protein for studying the effects of mechanical force. Fibronectin is found in connective tissue, such as the skin. In the skin, cells are suspended in the extracellular matrix — consisting of thousands of protein fibers that attach to cells at numerous points. These proteins connect with other proteins and hold the mass together — a sort of super glue for cells. It is the movement of these fibers and the resulting pull and push on the cells attached to them, that transmits force to the cells.
Vogel and colleagues ask these questions: What does force do to the fiber? How is force transmitted from the fiber to the cell? And how is force used to determine how the cell regulates the expression of certain proteins?
“We are very excited about this because we believe a new field is being born: non-equilibrium protein structure-function analysis. It’s very exciting to think about how nature regulates and controls function. We went from viewing the cell as a bag full of proteins a decade ago to a view of the cell as a dynamic place where proteins assemble and change under mechanical forces,” Vogel says.
This new field became possible only in 1997, with the technology that allowed researchers to see what happens when you grab either end of a protein and stretch, using tools such as atomic-force microscopy. They found that proteins rupture as stretching forces overcome energy barriers that stabilize the protein structure.
“Computer simulations are vital to learning how the structure changes,” Vogel says.
The computer simulations were done in collaboration with Dr. Klaus Schulten at the Beckman Institute , University of Illinois at Urbana-Champaign, and former UW graduate student Andre Krammer now at Molecular Simulations Inc.
“Only recently have people realized that mechanical tension — cells pulling on the matrix — is important,” says David Craig, a graduate bioengineering student and another of the paper’s authors.
Vogel’s lab is examining how mechanical force must be considered in the field of proteomics.
“In proteomics, you go from the genome, then to the protein structure, and from that, make a prediction about the protein’s function. But is that enough? Is it sufficient to only know the function in the equilibrium state?,” Vogel says. “We think there are a series of proteins that may have different structures, depending on how much force is applied and how it is applied. If that is so, then it adds additional dimension to the field of proteomics.”
To study this, researchers map out how a protein molecule is made up of smaller building blocks. Each of those building blocks is about 3 nanometers long (to get a sense of scale — there are 1,000 nanometers in a micron. A human hair is 50 microns wide). Several of these building blocks have numerous recognition sites, which can bind to other proteins or to cells. The paper in Proceedings looks at Type III domains within the fibronectin. The building blocks of Type III have the same physical structure, but their underlying amino acid sequences are very different.
UW scientists say that if you pull on fibronectin’s building blocks, hydrogen bonds are broken and one module in the chain ruptures: it stretches to its maximum. If you apply more tension, another module stretches, and so on. The paper also says that the fibronectin’s type III modules differ considerably in their mechanical stability, and that they can be stretched into a structural intermediate state before rupturing of the first big cluster of hydrogen bonds.
“This existence of a structural intermediate state may have significant functional implications,” Vogel says