Close Your IRB Application
Table of Contents
Before You Begin
Are you required to close your application?
| Review Type | Continuing Review Requirements |
|---|---|
| UW IRB Approved Studies | Application should be closed if the criteria for closure listed below are met. |
| Multi-institutional UW IRB Approved Studies
|
If the involvement of a relying institution has ended, submit a site closure to the IRB. This closes the SITE record while leaving the parent STUDY record open.
To close the study once the closure criteria listed below have been met, follow the Multi-site Continuing Review or Closure instructions. |
| External (Non-UW) IRB Review | Contact hsdrely@uw.edu to initiate the study closure process. |
| Exempt Determinations | Closure is not required. If your research is completed and you wish to stop receiving automated email notices, email hsdinfo@uw.edu to request that your exempt study be administratively closed. |
| Not Human Subjects Research, Not Research, UW Not Engaged Determinations | Applications with these determinations are considered completed and cannot be closed. |
Does your study meet the criteria for closure?
Criteria:
- All subjects have completed all study-related interventions and procedures, including any follow-up.
- You have obtained all private identifiable data and/or specimens from all subjects.
- You have completed your analysis of all private identifiable data and specimens, as described in your IRB application.
OR
- The study was never open for enrollment, no subjects were ever enrolled.
Common Concerns
Identifiers do not need to be destroyed in order to close an IRB application. IRB approval does not have to be maintained during the records retention period if the study procedures and analyses described in the IRB application have been completed.
You can still provide information to subjects about study results or other studies if the activities were approved as part of the application. When former subjects are provided with information about other studies, the IRB review of those other studies must include review and approval of the information and recruiting efforts.
The UW IRB application can be closed if the study is permanently closed to enrollment at all sites under the UW IRB’s review, all subjects under the UW IRB’s oversight have completed all research-related interventions and interactions, and no additional identifiable private information or specimens are being obtained by the investigators under the UW IRB’s oversight. This is true even if the UW study data have not yet been analyzed by the overall study statistical center/personnel under the oversight of another IRB.
Steps to Close Your IRB Approval
Step 1: Complete the Status Report Form
To request study closure, complete the Status Report form.
Step 2: Prepare Any Supporting Documents for Upload (If Needed)
Most closure applications only require the status report form, but you have the option to upload other documents related to the continuing review.
Any DSMB reports should be submitted separately using the reporting process.
Step 3: Complete the Continuing Review in Zipline
- Navigate to the study you need to renew and click Renew or Close under Next Steps
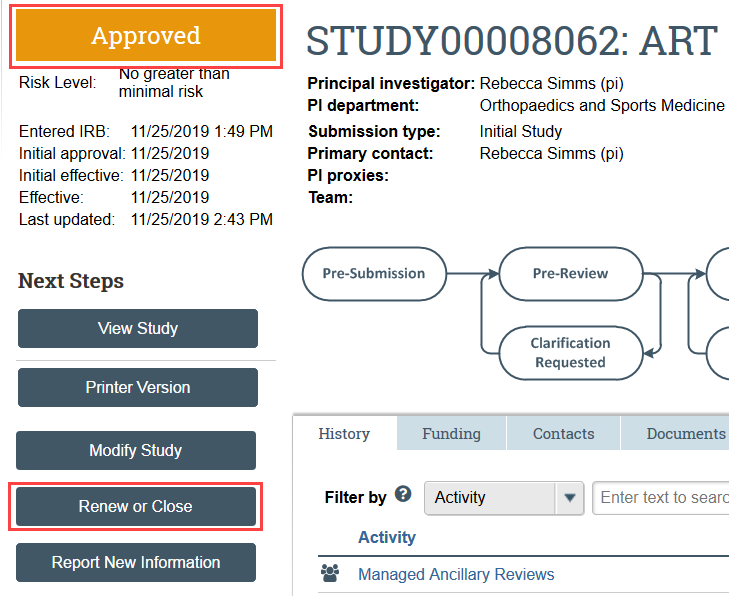
- Select Continuing Review as the purpose of the submission

- Complete the forms and upload the status report form and any supporting documents
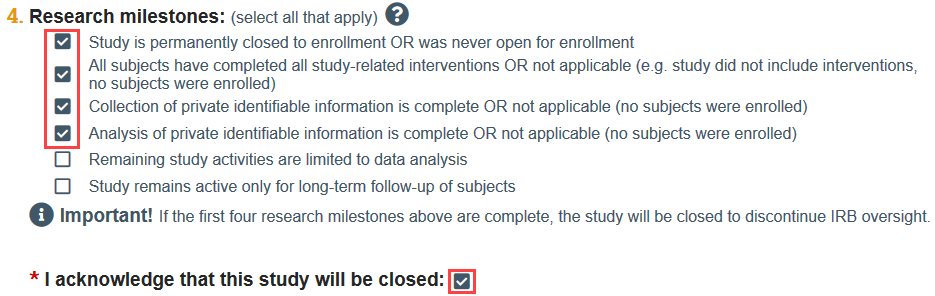
- If you indicate that the first 4 research milestones have been met, you will automatically be prompted to acknowledge that the study will be closed.
For step by step instructions:
Step 4: The PI or a PI Proxy must submit
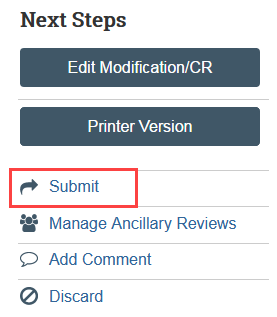
The Submit activity must be completed by the PI or a PI proxy once the continuing review is ready to go to HSD.
- Select Submit in the continuing review workspace and provide required verifications
What To Expect After Submitting
The continuing review transitions to Pre-Review state and is now in HSD’s queue for review.
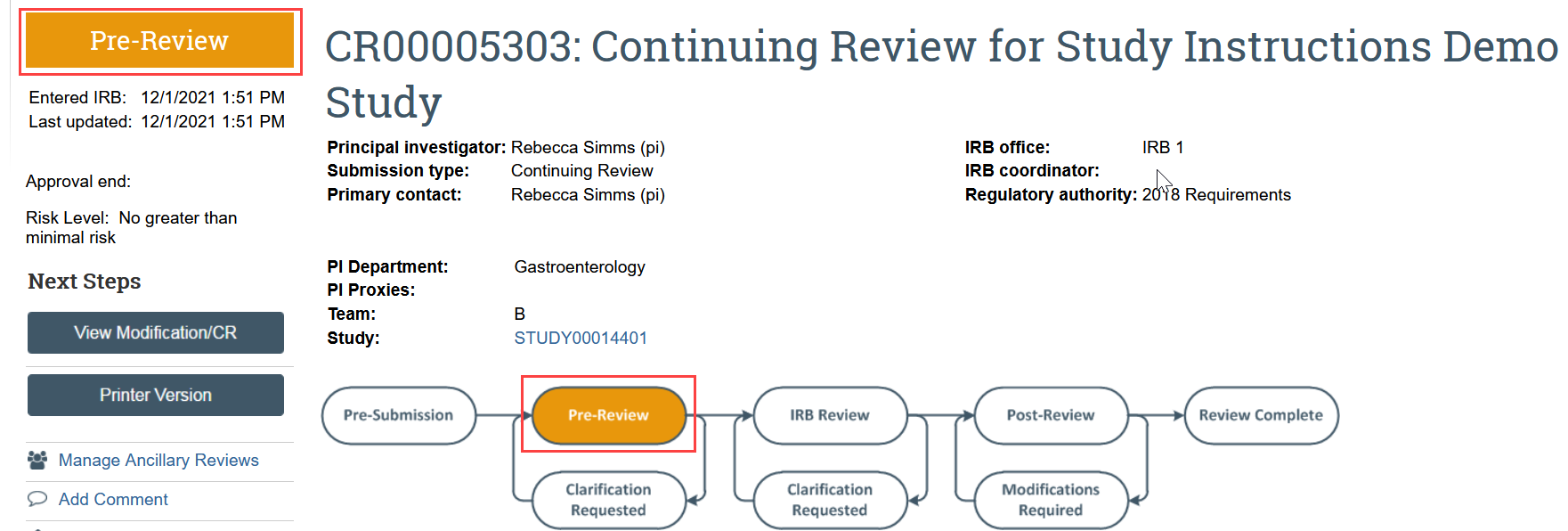
After the IRB has reviewed the application, the PI, any PI proxies, and primary contact will receive a notification that:
- The IRB has approved the closure; OR
- The IRB requires more information or a change before approving the closure
Visit Respond to HSD for more information on submitting responses.
After Closure
After the continuing review is approved, the parent study will be updated. For closure requests, the parent study status changes to Closed. The PI, PI proxy, and primary contact also receive a notification containing the Closure letter.
