After-the-Fact eGC1 Process
Updated 11/25/2025
An After-the-Fact (ATF) eGC1 includes both award agreements, which can be funded or unfunded sponsored projects and non-award agreements; which typically support a main sponsored project. Common non-award agreement types can include data use agreements, confidentiality, material transfer etc. Review more information on the variety of Agreement Types.
ATF eGC1s are used to document when a sponsor and UW PI have determined to establish sponsored program activity that:
- Lacks a proposal eGC1, or
- Needs a supporting agreement for an existing UW sponsored program, or
- Requires discussion with the sponsor prior to submitting a proposal such as confidentiality/non-disclosure agreements and teaming agreements.
Although not a proposal, an ATF eGC1 still requires the same campus unit review & approval. Additionally, OSP still provides the same institutional review.
Approval of an ATF eGC1 by OSP does not imply approval or acceptance of the agreement.
More information on:
The following instructions detail how to complete all types of ATF eGC1s.
ATF eGC1 Instructions
SAGE requires that you complete certain fields before it will allow you to route any eGC1 to OSP. Other fields, while not required by SAGE, are required by OSP and noted in these instructions.
eGC1 Details Page
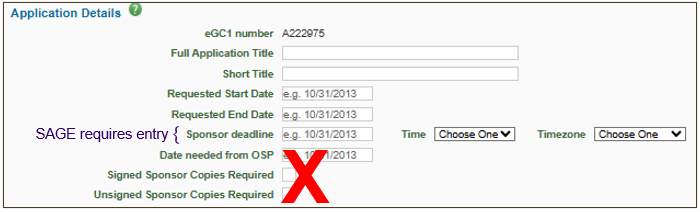
Application Details
System required fields on the Details page include: Full Application Title, Short Title, Start and End Dates, Application Type, Project Type, Sponsored Program Activity (SPA) Type, and Sponsor fields.
- Long and Short Titles: If the Agreement includes a project title, the eGC1 Full Application Title should match. Short Title can be anything meaningful for identification.
- Note: Industry Sponsored Clinical Trials Short Titles need to include the RedCap identifier number with the prefix RG and follow Getting Started eGC1 Review & Approval.
- Start and End Dates: Enter the Agreement period of performance if there is one. If not, enter “today” as the Start Date and enter an End Date at a time after that.
- Sponsor Deadline: System required field. OSP disregards this field for ATFs
- If the sponsor does have a deadline for award acceptance, include an explanation in Additional Information (eGC1 Certify Route section).
- Date needed from OSP: Disregard this field.
- Signed / Unsigned Sponsor Copies Required: Disregard these fields.
Cost Center Receiving Funding
see SAGE User Guide for details.
Application Type:
- ATF with Funding:
- Select the appropriate application type.
- Select the After-the-Fact Application=[YES].
- Non-Award Agreements (ATF without funding), select one:
- New Non-Award Agreement (new) for new agreements.
- Continuation of Non-Award Agreement (continuation) for an amendment that modifies the terms of an existing agreement.
- After the Fact application will default to [YES].
Project Details
- Project Type:
- ATF with Funding: Select the appropriate project type, ATF’s with funding are typically a grant or a contract.
- For help selecting the appropriate project type, review: How do I know which project type to select & why is it important?
- Non-Award Agreement (NAA): Select Contract
- ATF with Funding: Select the appropriate project type, ATF’s with funding are typically a grant or a contract.
- Sponsored Program Activity (SPA) Type:
- ATF with Funding: Select the appropriate Activity Type, based on the activities of the project.
- Non-Award Agreement (NAA): Select Other Sponsored Activity.
- For Pre-Clinical Trial Confidentiality Agreements (CDA): Select Clinical Trial, Non-Federal for the SPA Type
Sponsor
- Sponsor Details: Select correct Sponsor Name (or other party name).
- Include contact information for the “Sponsor” such as the contact name and email – OSP requires this information.
- Add the phone number if available.
- If readily available, provide any additional sponsor contacts in the notes field to prevent delays.
- Non-Award Agreement (NAA): For pre-clinical trial CDAs, if a Contract Research Organization (CRO) is listed in the agreement as the other party (e.g., ICON, WCT, IQVIA, PPD, Parexel) please enter (as the sponsor) the company the CRO represents.
- Include contact information for the “Sponsor” such as the contact name and email – OSP requires this information.
Originating Sponsor
- Does the funding originate from a different entity than the sponsor listed above (flow-through)?:
- ATF with Funding:
- If the funding originates from a different entity and is coming from a pass-through entity, select [YES].
- Otherwise, select [NO].
- Non-Award Agreements (ATF without funding):
- Select [No]
- ATF with Funding:
Additional Information for Existing Application or Award
- Non-Award Agreement (NAA): for non-award agreements (NAA) that support a main sponsored project include the related eGC1 number (i.e. sponsored project or previous NAA) enter it here.
- New ATFs and new confidential disclosure agreements (CDA): this field does not apply.
PI, Personnel, & Organization Page
Enter the name of the Investigator and any Senior / Key Personnel who will have the confidential discussion, handle the data or material, work on the project, etc.
Contacts & Assign Access
Enter the Administrative Contact (could be the same as the eGC1 preparer). This is the person we will contact with questions.
Abstract & RFA/RFP
OSP requires this information.
- Describe the goal(s), specific objectives, deliverables and/or summarize expected outcomes and purpose of the agreement in as much detail as possible.
- Include any special requirements, contextual information on what is happening, or conditions you think might help us understand your need.
- What will the UW receive?
- Is there UW background Intellectual Property (IP) involved?
Describing the purpose and goals in detail so that OSP understands the nature of the transaction can help prevent delays in processing your request.
Activity Locations
Required by the system (all three sections). Enter the location where the activity will occur.. For example: If you generated the data you are transferring in your lab at SLU, list the SLU location.
Budget & Fiscal Compliance/Cost Sharing
ATF with Funding: enter the amounts into the eGC1 Budget section or connect a SAGE Budget. We recommend creating and connecting a SAGE Budget.
Non-Award Agreement: Tab straight through both of these sections. There is nothing required here.
Non-Fiscal Compliance
All sections (Human Subjects, Animal Use, Environmental H&S, Equipment & Materials, Data & Technology) are required by SAGE. The answers here help OSP understand the terms that may need to be addressed in the agreement. In some cases additional approvals, licenses or tech transfer review might be needed before you proceed.
Confidential Disclosure Agreements/ Non-Disclosure Agreements (CDA/NDA) and the Human Subjects Section
CDAs and NDAs are most often used for preliminary discussions of a potential research project. Remember that, on the eGC1 for a CDA / NDA, these questions relate only to the information that will be disclosed in the preliminary discussion — not to the information that will be shared later for the actual project.
It is very rare for human subjects data to be disclosed in a preliminary discussion, and an unnecessary “yes” answer in this section will delay processing of your agreement.
Attached Documents
The UW prefers to use approved UW templates whenever possible.
- ATF With Funding: At the sponsor’s request, indicate you would like OSP to issue the UW Sponsored Research Agreement template to the sponsor. Or attach an editable DRAFT agreement from the sponsor.
- Non-Award Agreement:
- Data Use Agreements: we highly recommend the FDP DUA Templates.
- Non-Disclosure Agreements: If you want OSP to issue a UW Approved Non-Disclosure Agreement to the sponsor, include this request in the notes to OSP.
- Attach any background or back-up documents that will help OSP review.
- Examples: Communications with the other party, a protocol that relates to a data transfer etc.
Certify & Route
Complete all required areas of Certify & Route section.
Additional Information Section
While not required by SAGE, OSP requires the eGC1 preparer to enter details in the Additional Information section. The Additional Information section is where you tell OSP what it is you are trying to accomplish as well as indicate if you are requesting UW approved templates such as the UW Sponsored Research Agreement template or UW Approved Non-Disclosure Agreement.
Check for Errors
We encourage you to use Check for Errors when completing an eGC1 to find out which fields the system requires.

Route to Reviewers
Mark YES for the question: Is this application ready to submit to the sponsor.
ATF Approval Process
After an eGC1 is routed to OSP, a reviewer is assigned who confirms that the required info is included.
- Is it a proposal or actually an ATF?
- Is it complete and does it have all required elements?
- Is it a non-award agreement (NAA) or is there funding?
- Is there a request to issue UW Sponsored Research Agreement template or is an editable DRAFT agreement attached?
OSP approves complete ATF eGC1s and creates the next SAGE item to conduct a full agreement review.
Upon eGC1 approval for ATFs, OSP creates the Award Setup Request (ASR) or Non-Award Agreements (NAA) within 2 business days. Upon initiating an ASR, OSP will send the ASR to campus for completion according to ASR steps.
ASRs and NAAs are reviewed and processed in the order received and according to reviewer workload.
