Reviewing Follow-Ons
Table of Contents
General Process
For the most part, review for a follow-on submission (modifications, continuing review reports, and reports of new information) follows the same general process as review for an initial application. Refer to the page on reviewing initial applications for general steps.
See the sections below for more information on reviewing each type of follow-on submission. For combined modification/continuing reviews (MODCRs), simply see the sections for both modifications and continuing reviews.
Modifications
Review the Modification Workspace
The modification workspace is your access point for viewing the modification summary, viewing the draft changes to the study, and adding your review comments.
Generally, you will see the same tabs for the modification that you see for the initial application, including the History, Documents, and Reviews tabs.
![]()
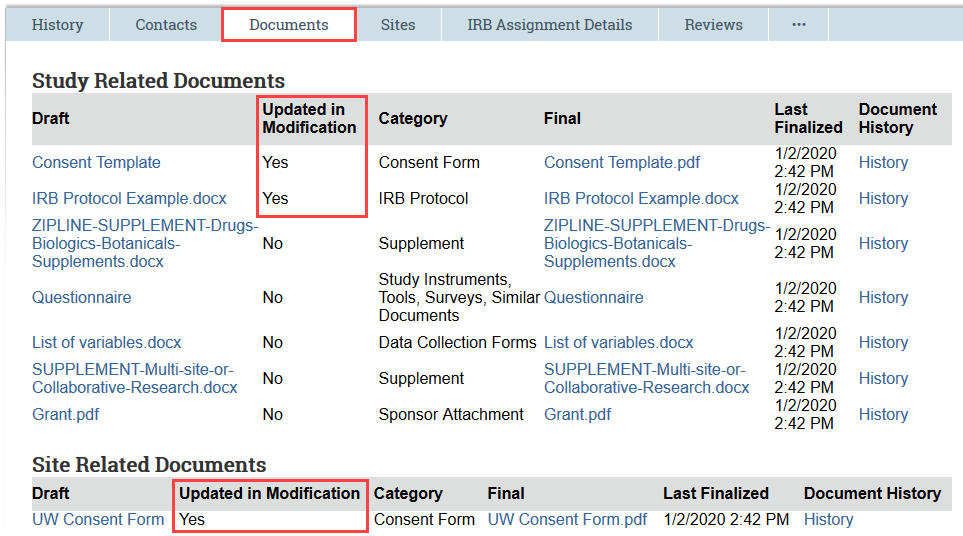
For modifications, the Documents tab indicates which documents were updated as part of the modification.
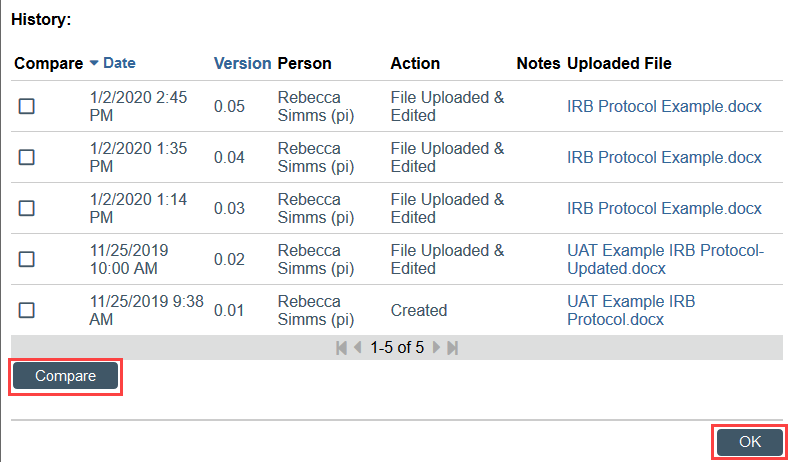
You can open the draft version to see any changes tracked by the study team, or you can use the Compare feature, available in the Document History, to compare any 2 versions of the document that have been submitted.
Any related reports of new information (RNI) are linked in the Related RNIs tab.
![]()
Review the Modification Summary
1. Click Review Modification/CR
2. Click the Modification Summary link in the lefthand navigator and review the summary
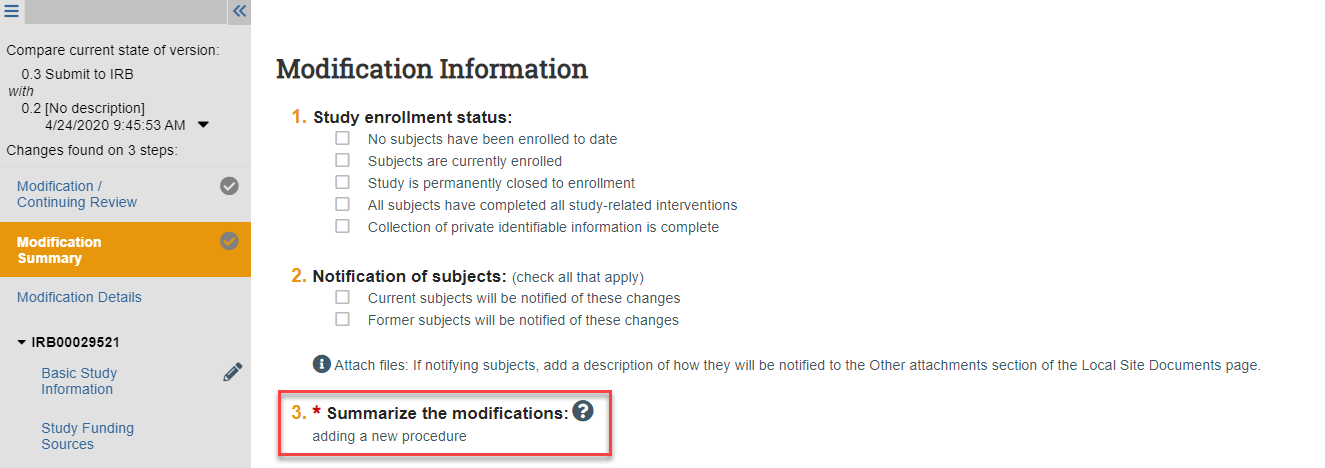
NOTE: If desired, you can use the “Above Section Has Been Reviewed” checkboxes to track your progress. No one else can see what you check, and it will persist over multiple sessions viewing the application.
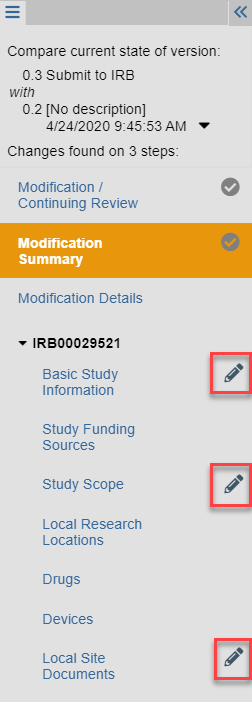
Review the Modification Changes
After you click Review Modification/CR, Zipline automatically compares the modified version of the application to the most recent version.
The pencil icon in the Lefthand Navigator indicates which application pages have been changed.
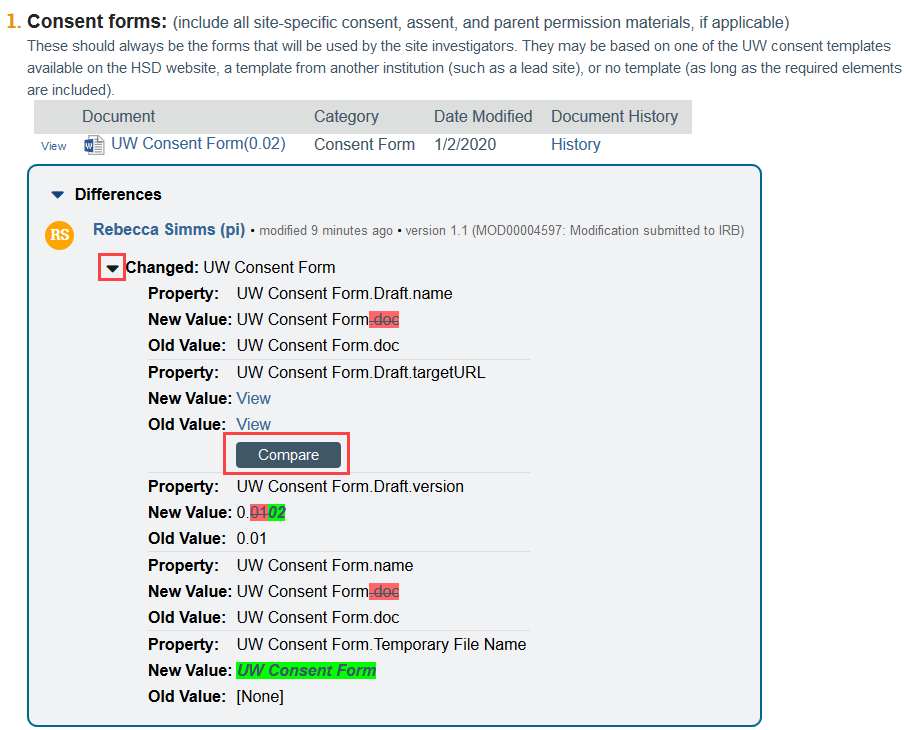
If a document has been updated, expand the box highlighting the change to run Compare mode.
Review the Study Workspace
A link to the study is available in the Modification workspace. You can view other open follow-on submissions in the study workspace.

Continuing Review Reports
Review the Continuing Review Workspace
The continuing review workspace is your access point for viewing the continuing review report, viewing additional information about the currently approved version of the study, and adding your review comments.
Generally, you will see the same tabs for the continuing review that you see for the initial application, including the History, Documents, and Reviews tabs.
![]()
For continuing reviews, the Documents tab contains the most current versions of all of the study documents, including the IRB protocol and any consent forms. You’ll also find any documents that were attached to the continuing review submission, including the status report form that researchers must complete and upload.
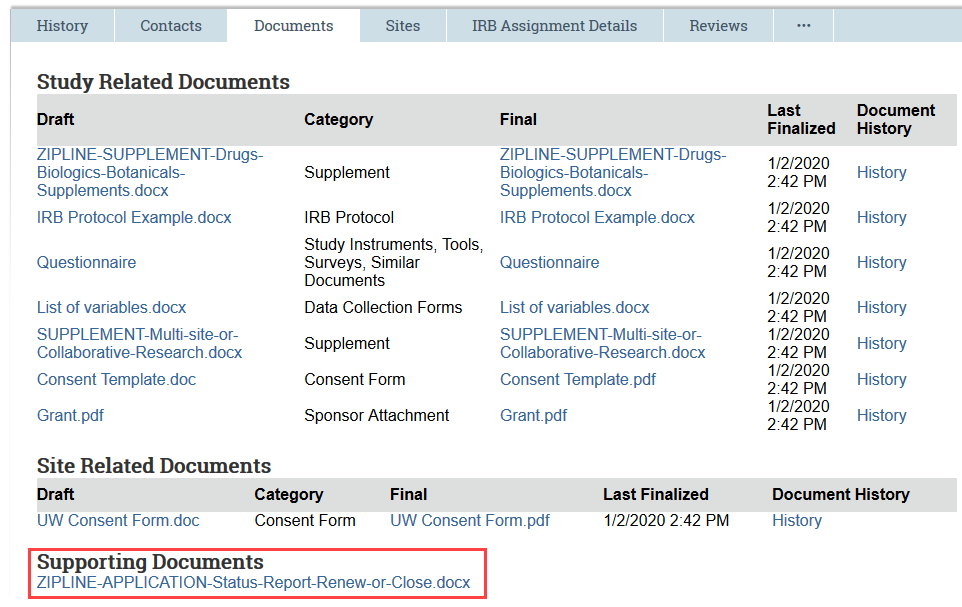
Review the Continuing Review Report
1. Click Review Modification/CR in Next Steps

2. Scroll through the form to view continuing review information
3. Click the link for the attached status report to open and review
Review the Study Workspace
A link to the study is available in the Continuing Review workspace. You can view other open follow-on submissions in the study workspace.

Reports of New Information
Review the RNI Workspace
The RNI workspace is your access point for viewing the report of new information and adding your review comments.
The History, Documents, Related Submissions, and Reviews tabs are available for RNI.
- The RNI Documents tab does not contain any study documents such as the IRB protocol or the consent forms. The RNI Supplement and other documents attached to the RNI currently do not display in this tab- this is a known issue.
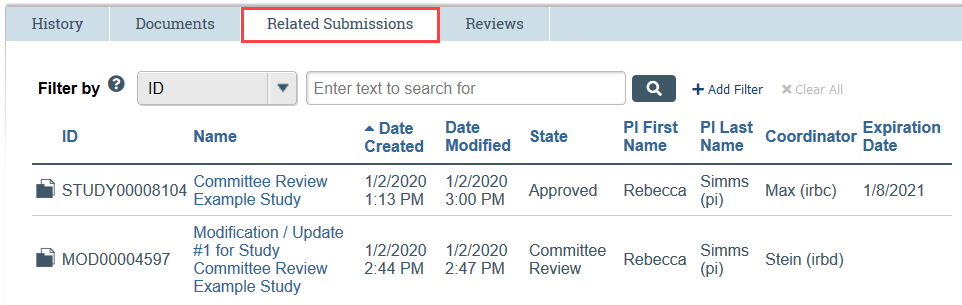
- The Related Submissions tab displays a list of any studies, sites, or modifications that have been associated with the RNI in Zipline. RNI can be associated with no studies in Zipline or with multiple studies in Zipline- it will depend on the situation. Simply click the linked name to open the related submission.
![]()
Review the RNI Report
1. Click Review RNI in Next Steps
2. Scroll through the form to view RNI information
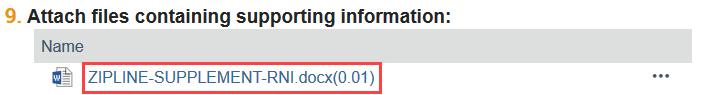
3. Open any attached documents, such as the RNI supplement
Review the Study Workspace
Navigate to any related studies using the Related Submissions tab. Study documents such as the IRB protocol and consent forms must be accessed via the related study or modification workspace.