Research Involving Kaiser Permanente Washington
Research involving two institutions normally requires review by each institution’s IRB. To avoid this “dual review” and the extra work it requires from researchers, Kaiser Permanente Washington (KPWA) and the UW IRB have a reliance agreement that describes the circumstances in which one of the IRBs will conduct the review on behalf of both institutions.
The Kaiser Permanente Interregional IRB (KPiIRB) is the IRB for KPWA as well as several other components of Kaiser Permanente. This information applies only to research involving Kaiser Permanente Washington. It does not apply to other components of Kaiser Permanente outside of Washington.
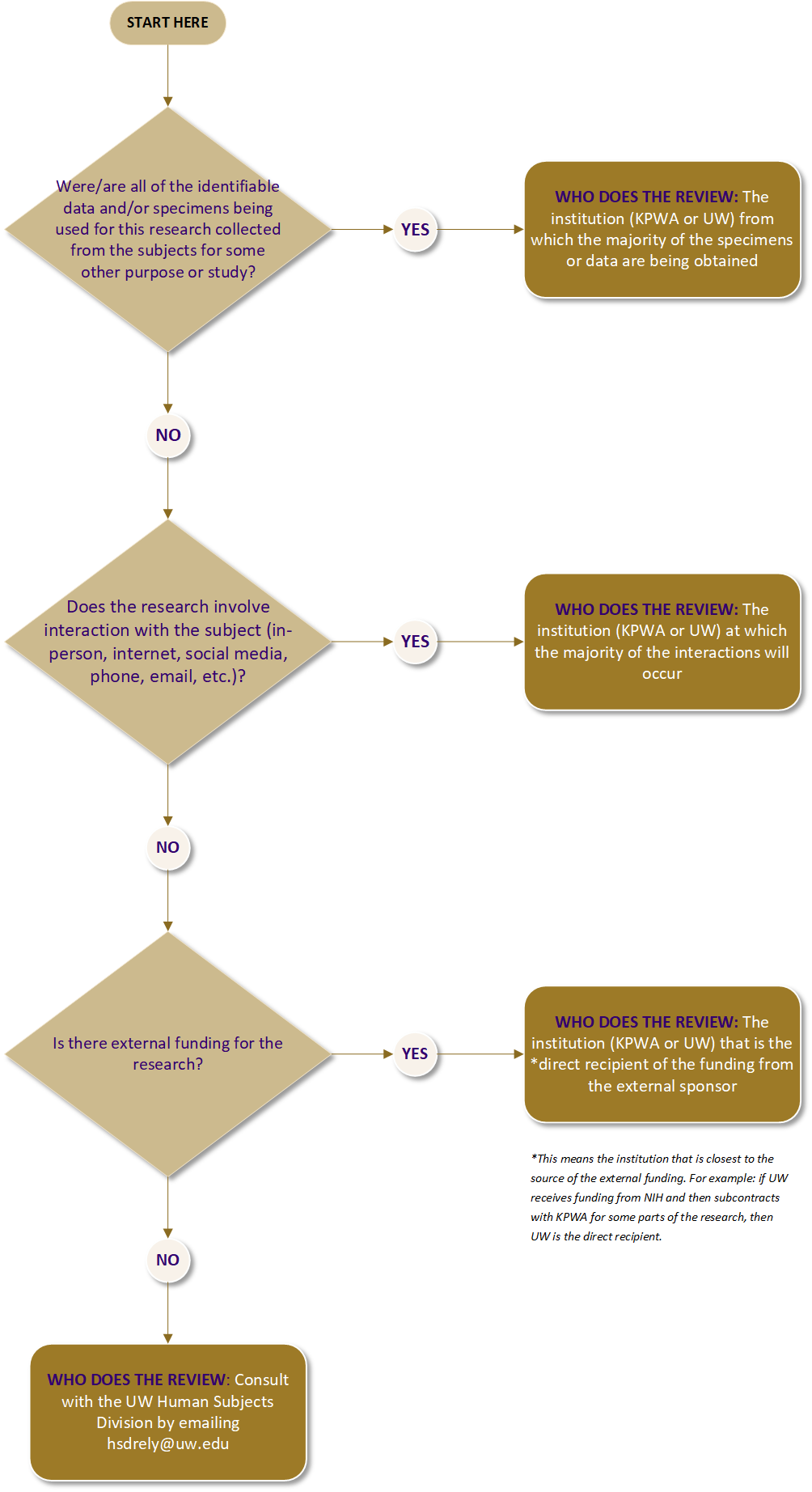
Use the flow chart below to determine which IRB should do the review. If you have any questions, consult HSD at hsdrely@uw.edu or the KPiIRB at kpinterregionalirb@kp.org.
Before you begin
- Assess whether both institutions are engaged in the research. If only one institution is engaged, you should submit to that institution’s IRB. Consult with HSD at hsdrely@uw.edu to identify whether UW is engaged.
- Assess whether another IRB is the right option. For example, most industry-sponsored research at UW should be reviewed by an independent IRB (for example, Advarra) and some cancer-related research should be reviewed by the Fred Hutch IRB or the NCI IRB. Review the information about identifying the correct IRB if you are unsure.
What to do if KPiIRB will do the review
- Submit an External IRB Application in UW’s Zipline system to request HSD’s formal authorization for KPiIRB to review the research. HSD will evaluate your request and provide you with a written authorization letter.
- The KPWA investigator should prepare and submit an application to the KPiIRB using KPiIRB’s forms and process. Be sure to attach the HSD authorization letter; the KPiIRB will not review the application on behalf of UW without this letter.
- After the KPiIRB has approved the UW’s participation, send the approval letter to hsdrely@uw.edu.
- Obtain all other applicable ancillary reviews (consult the Checklist of Researcher Responsibilities for Externally Reviewed Studies) from the usual UW offices and KPWA as needed. Examples: Financial Conflict of Interest (FCOI) review; Radiation Safety review.
What to do if UW will do the review
- Submit a UW IRB Application in the Zipline system. Be sure to:
- Describe all involvement of KPWA in your application (the Zipline SmartForms as well as the IRB Protocol form that is uploaded).
- Follow the instructions for the UW Reviewing for Other Institutions pathway.
- On the Study-Related Documents SmartForm: Upload a completed SUPPLEMENT: Multi-site or Collaborative Research to Question 3.
- Submit a request to rely on the UW IRB to the KPiIRB using KPiIRB’s forms and processes. KPiIRB will evaluate your request and provide you with written authorization for the UW IRB to do the review. Include this written authorization in your application to UW or email to hsdrely@uw.edu.
- The UW IRB will review and approve the overall protocol. This is not yet approval for the involvement of KPWA. After the UW IRB has approved the overall protocol, HSD’s Reliance Team will work with you to approve the addition of KPWA to the UW application.
- After UW IRB approval has been obtained, you are responsible for:
- Informing the KPiIRB of UW’s approval for KPWA.
- Informing KPWA investigators about any conditions of the IRB approval.
- Informing KPWA investigators about their obligation to comply with UW IRB reporting requirements about noncompliance, complaints, problems, etc., although such reports must be submitted by you to the UW IRB.
- KPWA investigators remain responsible for obtaining any applicable ancillary reviews from KPWA (for example, Financial Conflict of Interest (FCOI) review, Radiation Safety Review).
KPiIRB or UW IRB Decision Tree