Modifications to UW Reviewed Studies
Review Modify the Approved Study for an overview of the modification process, including which review types require modifications.
How to Modify An Approved Study
Step 1: Edit Approved Study Documents and Prepare New Documents
Always use the currently approved version of the document that you need to edit, accept any previous tracked changes, and make sure tracked changes are enabled before making edits. Make sure you update any document dates and version numbers too!
Previously Approved Documents: In the Documents tab, open the Final version of the document to be edited
- FOR CONSENT FORMS: Because the final version is a PDF, select the draft version in Word instead of the final version. In some cases, this document may contain tracked changes and comments that have been accepted in the final version. If so, use the review features in Word to accept all the changes and remove any comments, and use this clean document as a starting point for your revisions.
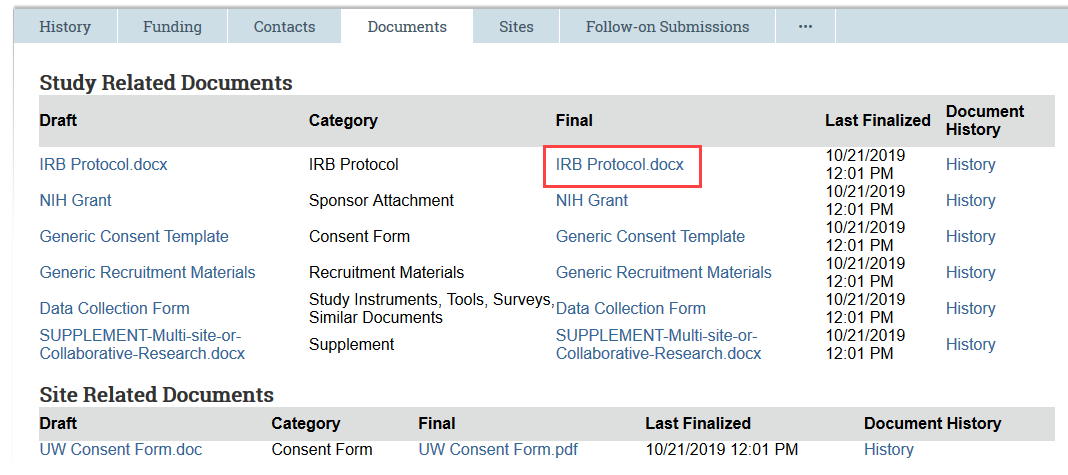
- Make document edits and save the document to your computer
To aid in HSD’s review:
- Make sure Track Changes is enabled before making changes
- Update the date and version number in the header/footer of your document as needed
Step 2: Create Zipline Modification
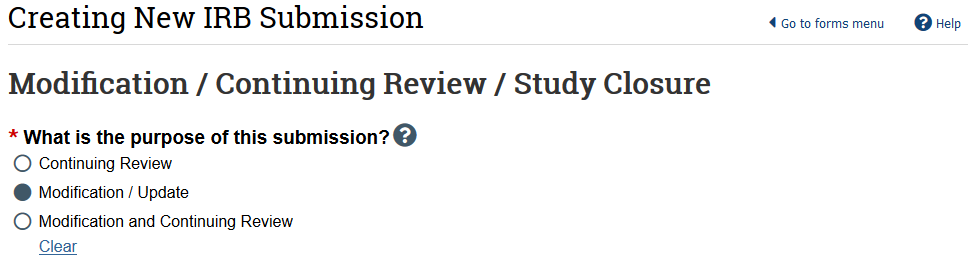
- In the study workspace, select Modify Study

- Select Modification/Update as the purpose
- HSD recommends creating 2 separate submissions instead of a combined modification and continuing review
- Select “Other parts of the study” as the modification scope (select study team member information if you also need to update Zipline access)

Step 3: Complete the Modification Information Form
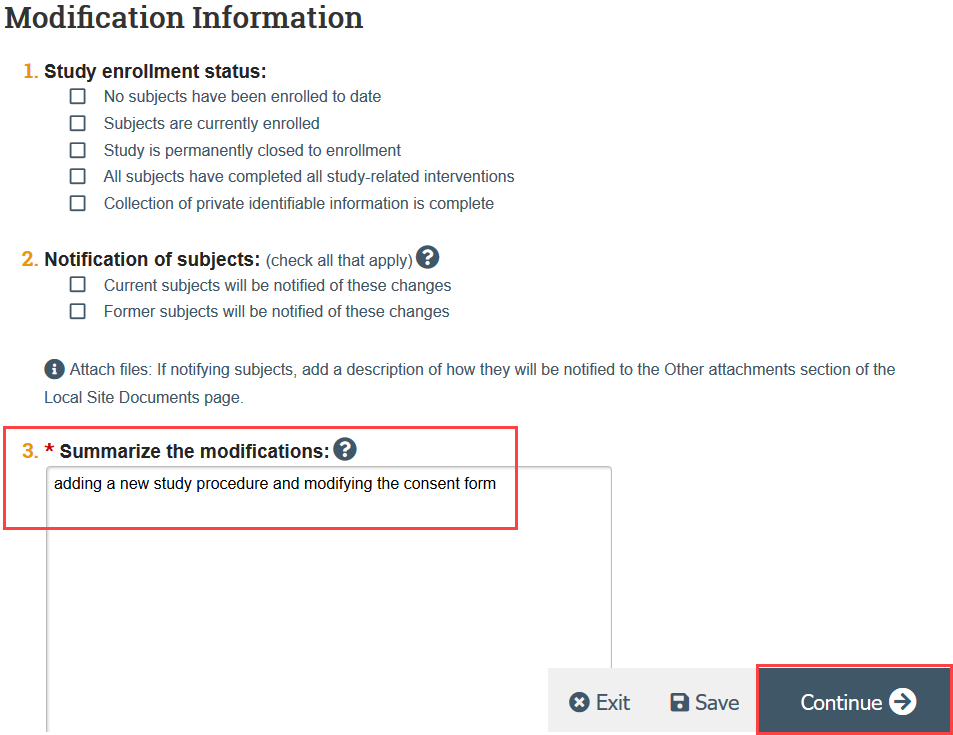
- Complete the Modification Information SmartForm page, including a summary of your modifications and click Continue
- If the modification is in response to a Report of New Information (RNI), note this in the modification summary and link the modification and RNI in the RNI workspace
Step 4: Update the Zipline Application, including Uploading Revised Documents
- You are now in a draft version of the study SmartForm and should be on the Basic Information page. Update the draft version of the study by making all needed revisions to the Study SmartForms and uploading any revised or new documents.
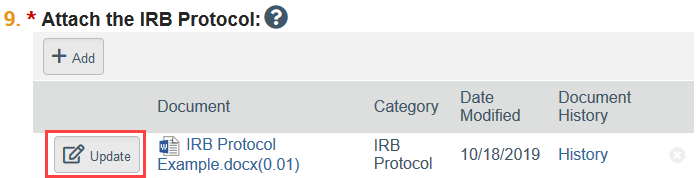
To Update a Document:
- Click Update
- Select the revised file from your computer
- Click OK in the Edit Attachment window
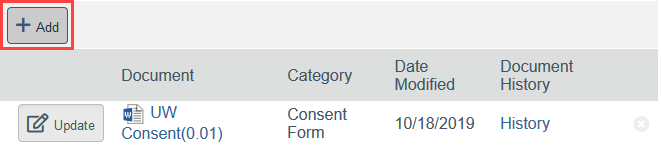
To Add a New Document:
- Click Add
- Select the new document from your computer
- Click OK in the Edit Attachment window
-
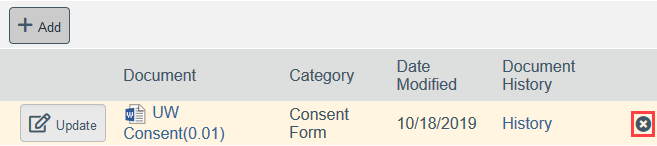
- To Remove a Document:
- Click the X by the document
- To Remove a Document:
- Click Save and Exit when you are finished editing the modification
Step 5: Review Modification for Completeness
Checking the application for errors and omissions helps you to include all the relevant information, which is essential for receiving a timely review of your modification. Automatic system error checking identifies any omitted answers to questions that are required for every application. A red asterisk (*) indicates required questions.
HSD also recommends looking over the forms to see what you may have missed, especially:
- Questions that are relevant to your study but are not required for all studies (no asterisks)
- Documents that should be attached
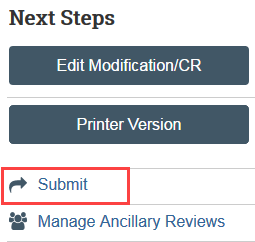
Step 6: Principal Investigator (PI) or PI Proxy Must Submit
The Submit activity must be completed once the modification is ready to go to HSD. This activity must be completed by the PI or the PI Proxy.
- Select Submit in the modification workspace and provide required verifications
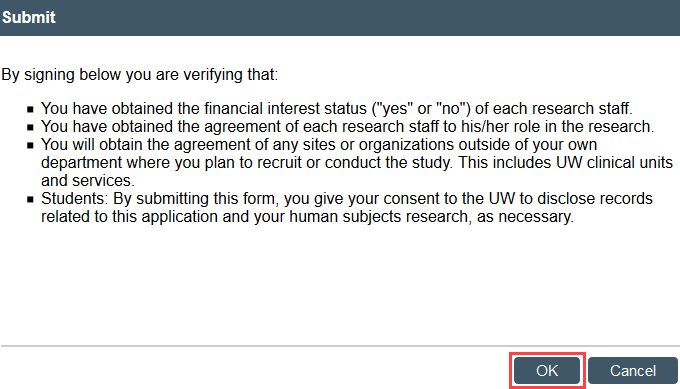
The study transitions to Pre-Review state and is now in HSD’s queue for review.
