Zipline FAQs
See the topic pages below to access many frequently asked questions about Zipline. If you are unable to find the answer to your question, contact the HSD team that reviews for your department or hsdinfo@uw.edu for help.
Getting Started
- What is the review process in Zipline?
- What is a SmartForm? What do all these terms mean?
- I just created a Zipline account. Why can’t I see any studies?
- What do I need to know about uploading study documents?
- Why hasn’t IRB review started yet?
- I can’t find my PI/faculty advisor/study team member in the system. How do I find them?
- I’m not sure how to answer a question on the SmartForm or the IRB Protocol Form. What should I do?
External (Non-UW) IRB Studies
- The sponsor/coordinating center has sent me updated study documents for an externally reviewed study. How do I update this in Zipline?
- When I need to modify an external submission, what materials should I update?
- How do I update or modify an external submission in Zipline?
- I can’t find the name of the IRB I want to rely on in Zipline. What do I do?
- What is the Correspond with sIRB activity?
Single IRB
- What qualifies as another site or institution on my project?
- What does applying for UW Single IRB (sIRB) review look like in Zipline?
- How is HSD going to communicate with me about SITEs in Zipline?
- How do I make site-specific Modifications, including changing a SITE PI?
- How do I close a SITE in an active study?
- What is the Correspond with Site activity that shows up for SITE applications?
Getting Started
What is the review process in Zipline?
At the highest level, all Zipline submissions follow the same basic workflow.
The actions that you can take on a study depend on the study state. Zipline review is like a tennis match- when the application is with HSD (in Pre-Review, IRB Review, or Post-Review), then you cannot make edits. Similarly, when the application is with the study team (in Pre-Submission, Clarification Requested, or Modifications Required), then HSD cannot submit review to push the application forward in the process.
Once the PI or a PI proxy submits the application, it moves from Pre-Submission to Pre-Review. From there, HSD can request clarification from the study team as many times as necessary until review is complete. When HSD sends the review outcome letter, the study transitions to review complete.
The system shows the diagram below when you view an individual submission and shows the current state in orange.

The submission state indicates where the submission is in the IRB process workflow.
What is a SmartForm? What do all these terms mean?
The SmartForm is the series of forms that Zipline presents you when viewing or entering study information. Study documents, such as the IRB protocol and consent materials, are uploaded to the SmartForm. It is called a SmartForm because the questions and number of forms included may change based on the answers you provide.
For a list of Zipline terms with definitions, visit the Zipline Glossary.
I just created a Zipline account. Why can’t I see any studies?
When you log in to Zipline, you are only able to see studies that you have been given permission to view or edit. If you only need to view a study, a study team member who can access the Zipline study may add you as a study guest. If you need to edit the study and/or act as a PI proxy, you must be added to the Local Study Team Members page on the Zipline application. Review Manage Study Access for more information.
What do I need to know about uploading study documents?
- Study documents should be uploaded in Microsoft Word format whenever possible because they may need to be edited and changes may need to be tracked over time. UW offers Microsoft Office to UW students, faculty, and staff at no charge.
- Study documents in Zipline should be uploaded to the appropriate locations in the SmartForm application. This matters because the upload location determines how the document is labelled and handled in Zipline. For example, only consent materials should be uploaded to the Consent Materials section so they are watermarked with HSD’s approval stamp and labelled appropriately. For detailed information about what documents should be uploaded and where they should go in the SmartForm, review Step 4 on the Apply for Review page.
- When uploading a revised version of a document, it’s important to use the “Update” button instead of the “Add” button so that Zipline can archive the previous version for HSD reviewers.
Why hasn’t IRB review started yet?
If the item is still in Pre-Submission, you have not yet submitted the item to HSD. After you have completed the application forms and SmartForms, the PI or PI proxy must click the Submit button for IRB review to begin.

If the item is in Pre-Review, it is in HSD’s queue for review. Contact the team that reviews for your department for more information.
I can’t find my PI/faculty advisor/study team member in the system. How do I find them?
The person who creates the study in Zipline is automatically listed as the PI and can be changed by clicking the “ellpsis” button next to the PI question on the Basic Information SmartForm (the first web form of the application).
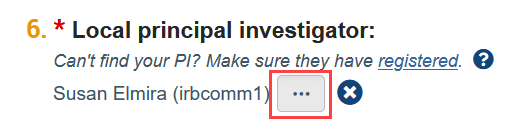
If the PI is not listed in the selection window that appears, it means they have not yet registered in Zipline. Similarly, if the faculty advisor is not listed in the selection window that appears when adding an ancillary review or a study team member is not listed in the selection window on the Local Study Team Members page, it means that they have not yet registered in Zipline. Please visit Account Creation and Management for more details. Once a person has registered, they will show up as a selection option in Zipline.
I’m not sure how to answer a question on the SmartForm or the IRB Protocol Form. What should I do?
For SmartForm questions, check the related help bubble for more guidance about how to answer the question.
In the IRB Protocol, start by taking a look at any instructions or examples embedded within the form to help answer your questions.
If you are still unsure how to proceed, contact the HSD Team that reviews for your department or hsdinfo@uw.edu for additional guidance.
Study Team
How do I assign someone to act as a PI proxy in Zipline?
The Principal Investigator (PI) can assign a proxy who can perform all actions normally restricted to the PI on a study, such as submitting the application to HSD, submitting modifications and continuing review, and copying a previously created study. PI proxies are assigned on a study by study basis. A proxy receives email notifications from the IRB system whenever the PI and primary contact receive them.
Only people listed on the Local Study Team Members SmartForm page in Zipline can be added as PI proxies. If the study is already approved and you need to add someone to the Local Study Team Members SmartForm, submit a modification to update the study team.
Once the study team modification is approved, the PI can update the PI proxy list for the study.
Do I need to add my entire study team on the Local Study Team Members page in the SmartForm application?
No. Only those who need viewing and editing privileges and those who will be a PI proxy must be listed on this page.
The study coordinator changed. What do I need to do?
To change Zipline access only: Submit a study team member modification to update who should be able to access the application.
If study team qualifications or other documents, such as consent forms, are also changing: Submit a modification to change both the study team member page and other parts of the study. Visit Modifications to UW Reviewed Studies for more information.
The PI is changing. What needs to happen with the Zipline application?
The new PI will not have access to the Zipline application until a study modification updating the PI is approved. If possible, we strongly recommend having the former PI designate a PI proxy to ensure that someone on the study team is able to submit a modification and respond to any questions from HSD. If the former PI is no longer available, contact the HSD team that reviews for your department for assistance.
Funding
What do I do if my sponsor is not listed as a funding organization in Zipline?
You can search for part of the name of your funding source using the % symbol (e.g., if you’re looking for the ‘Patient Centered Outcomes Research Institute (PCORI)’ you could search ‘%Patient’ ‘%Patient-Centered’ ‘%Research Institute’, etc.).
If your funding is internal to the University of Washington (e.g. internal department funds), the funding source will not be listed in Zipline. Select instead “OSP Not Involved”. Write N/A for the Sponsor’s Funding ID and Grants Office ID.
If your funding is a gift disbursed through the University of Washington, the funding source will not be in Zipline. Select “Gift through the UW” instead. Write N/A for the Sponsor’s Funding ID and Grants Office ID.
If your funding source is not internal to the UW, is not a gift, and does not appear on the list, contact HSD staff at hsdinfo@uw.edu before you complete your application, so it can be added to the list for you to select.
I added a funder in Zipline, but now I need to make revisions. How do I edit the funding source?
Once you have added a funder, click on the Funding Source name to open the specific funder information.

A slide-in will appear and you can edit the funder information from there.
Click OK when you have finished editing.
Responding to HSD
I can’t find the Submit/Submit Response button. How do I send the application to HSD for review?
Look for the ‘Submit’ or ‘Submit Response’ button on the left hand side of the study workspace under Next Steps. Only the PI or PI Proxy has the ability to submit the study or to submit a response to HSD requests for clarification. For instructions on how to add a PI Proxy to your study, see Assign PI Proxy.
I submitted my application, but I forgot something. How do I make a change right now?
If the application was just submitted, you can withdraw your study during Pre-Review to change something you have forgotten. However, you will have to re-submit your study after you have made the changes, and if HSD staff started review, it will have to start over when the application is resubmitted.
If review has started, contact the assigned IRB coordinator. The IRB coordinator can request changes to push the application to you for further editing.

Should I use a comment to contact HSD about my application?
In general, HSD does not recommend using the Add Comment button if you need to contact HSD staff about your application.
![]()
HSD staff do not routinely monitor applications for comments. If you have a question about your application, the best way to ensure a prompt response is to email the team that is assigned to your study. If no team is assigned, email hsdinfo@uw.edu.

The Add Comment button cannot be used to respond to a clarification request from HSD because it does not push the review forward in the system. If the application is in Pre-Submission or Clarification Requested state and you need to push it to HSD so that the review can move forward, use the Submit or the Submit Response button. This must be completed by the PI or a PI proxy.
Modifications
Why are there 2 types of modifications?
Zipline asks you to indicate the modification scope when you are submitting a new modification. The options are “Study team member information” and “Other parts of the study.” You can select one or both options. Zipline will not let you submit a second modification with the same scope until the first one is discarded or approved.
Study team member modifications are intended to be quick administrative study changes that simply update who is able to view and edit the study record in Zipline.
For any other study change, including changes to the study roles, you must indicate “other parts of the study” for the modification scope. This triggers the standard IRB review process.
What should I do if I selected the wrong modification scope?
The page that indicates the modification scope is not editable once you’ve progressed past that page.
Option 1: Discard the modification that has been created and start again. This option is only recommended if you have not begun making your changes in the modification or if you selected the incorrect modification scope initially.
Option 2: Create a second modification and check the needed modification scope box there. Zipline allows a second modification to be created if the scope is different from the modification currently in review.
Faculty Advisor Review
My faculty advisor can’t submit ancillary review. What should I do?
First, make sure that the faculty advisor was added as an ancillary reviewer. In the study workspace, click Manage Ancillary Reviews under Next Steps. If you do not see your faculty advisor as a reviewer, you must add them. See Add Faculty Advisor Review for more information.
If your faculty advisor was added as an ancillary reviewer but does not see Submit Ancillary Review as an option for your study, make sure that your faculty advisor is not listed on the Local Study Team Members page in the study SmartForm. If your faculty advisor is listed on this page, removing them will correct the issue. If your study application is not in an editable state, contact the assigned IRB coordinator or the HSD team that reviews for your department for assistance.
External (Non-UW) IRB Studies
The sponsor/coordinating center has sent me updated study documents for an externally reviewed study. How do I update this in Zipline?
It is likely that you do not need to update these documents in Zipline. HSD requires minimal changes to external submissions. Review this list of changes that require you to update information in Zipline.
When I need to modify an external submission, what materials should I update?
When making updates to an external submission, make the minimal changes necessary. For example, if you are changing the UW PI, previous versions of a study protocol or uploaded draft consent materials should not also be updated. You may be asked to remove these updated documents by HSD staff. For assistance with your specific submission, please contact hsdrely@uw.edu.
How do I update or modify an external submission in Zipline?
There are two types of submissions that are used to make changes to external submissions in Zipline. One is a Study Update and the other is a Site Modification. For certain submission types making particular changes may require both a Study Update and a Site Modification. For assistance with your specific submission, please contact hsdrely@uw.edu.
I can’t find the name of the IRB I want to rely on in Zipline. What do I do?
If your external IRB is not on the list, contact HSD staff at hsdrely@uw.edu before you complete your application. Please note that the UW does not rely on international IRBs. When you reach out to hsdrely@uw.edu, HSD staff will assess whether your proposed external IRB can be added to the list for you to select.
What is the Correspond with sIRB activity?
Do not use the Correspond with sIRB activity. If you use this activity, your message will not reach any recipients. The Correspond with sIRB activity is built in by our system vendor and is meant to facilitate communication between the reviewing IRB and UW. However, at this time, HSD does not use the Correspond with sIRB activity to communicate with contacts at other institutions.
Single IRB Studies
What qualifies as another site or institution on my project?
In Zipline, selecting “Multi-site/collaborative” on the Basic Information SmartForm page instead of “Single site” means that there are other institutions that will be involved in conducting the research with human subjects.
This question in Zipline is not about research locations where you or other UW study team members will conduct activities, but rather indicates that there are other non-UW sites where non-UW investigators will perform research activities. For example:
- A UW researcher visits an introductory course at Seattle Pacific University to recruit SPU students to participate in a study about burnout in college students. No one from SPU will obtain consent, administer study interventions, or collect or otherwise obtain identifiable data. This is a single site (UW-only) study.
- A UW researcher collaborates with investigators at UCLA and Johns Hopkins on a novel mental health intervention for college students. All three institutions will follow an established protocol to conduct the research; there is a lead investigator and study team at each site. This would be a Multi-site/collaborative study.
Review our Engagement Guidance or reach out to hsdrely@uw.edu for more information.
What does applying for UW Single IRB (sIRB) review look like in Zipline?
The STUDY submission provides the primary description of the study and of the local (i.e., UW) involvement in the research. Like an application for a UW-only submission, it includes several SmartForms and the IRB Protocol form, which should describe the overall or generic study. It should also include a Multi-Site Supplement, which will list the other engaged sites and the site lead investigators. Other study documents that might be uploaded include: questionnaires used at all sites; a generic consent form; a grant application; and any appropriate Supplements (e.g., the Drug or Device supplement). For detailed instructions review Submitting New Sites.
A SITE submission is created by HSD staff for each non-UW institution (“site”) involved in the research. Do not create your own SITEs in Zipline. SITE submissions are linked to the study submission and are listed under the “Sites” tab in the overall STUDY workspace. The SITE submission SmartForm is very short and collects site-specific information. After HSD staff create the site, they will send instructions to the UW team requesting site-specific materials including a SUPPLEMENT Site Application and other documents such as a site-specific consent form. The documents submitted in the study submission (such as the IRB Protocol form) are automatically displayed in the site submission and do not need to be completed or submitted again.
How is HSD going to communicate with me about SITEs in Zipline?
For initial SITE reviews, the SITE is created by HSD staff. HSD staff will inform you when the SITE is created and provide instructions via email or Zipline comment. The SITE will remain in the “Invitation Pending” status through the entire initial review. Zipline does not allow HSD to request clarification or for you to respond in the SITE submission. Instead, HSD will communicate questions or revisions to you via a Comment. Once you have responded to the request, you will use the “Add Comment” activity to notify the HSD that the SITE is ready for review. When the SITE is ready for review HSD will issue SITE-level approval, which includes a site-specific approval letter, and the status will change to “Active.” If the SITE cannot be approved or is removed from the study prior to approval for any reason, the status will be changed to “Inactive.” For detailed instructions and screenshots, review Submit SITE and Communicate with HSD.
SITE-level Modifications are created within the SITE by the study team following the standard Modification creation and submission process. Again, Zipline does not allow HSD to request clarification or for you to respond. Instead, if additional information or revisions are needed, HSD will “Withdraw” your SITE Modification and add a Comment to explain. You will be notified that the SITE Modification has been withdrawn and the submission state will be “Pre-Submission”. Once you have responded to the reviewer’s request, the PI or PI Proxy must hit “Submit” to change the state of the SITE Modification back to “Pre-Review.” Use the “Add Comment” activity to notify HSD that the SITE is once again ready for review. This withdraw and resubmit cycle will continue until the HSD can complete the review of the SITE Modification. When approval is issued, the SITE Modification state will change to “Approved”. For detailed instructions and screenshots, review Communicating with HSD During Site Modification Review.
How do I make site-specific Modifications, including changing a SITE PI?
Many changes to SITEs must first be approved at the STUDY or overall protocol level. For example:
- A change in the Site PI would first require a change to the Multi-Site Supplement which is located in the STUDY submission
- An expansion of the study scope to include children at one site would require the IRB to make STUDY level determinations regarding a new vulnerable population
- A new request for access to protected health information without prior consent would require the STUDY level consideration of a HIPAA waiver
Once any STUDY level Modifications have been approved, any site-specific changes should be made with a SITE Modification.
How do I close a SITE in an active study?
Submit a Modification to the overall study. This Modification should include an updated Multi-Site Supplement (using tracked changes) to remove the site that is no longer active. In the Modification summary, explain the reason for the site closure. HSD will review the Modification and ask for any additional information or documentation needed. Once everything is received, HSD will approve the Modification and de-activate the SITE. There is no need for a SITE-specific submission to close a SITE. HSD does not provide a SITE-specific closure letter in most cases.
What is the Correspond with Site activity that shows up for SITE applications?
Do not use the Correspond with Site activity. If you use this activity, your message will not reach any recipients. The Correspond with Site activity is built in by our system vendor and is meant to facilitate communication between the lead institution (UW) and any relying institutions. However, at this time, we do not use the Correspond with Site activity to communicate with contacts at other institutions.
RNI
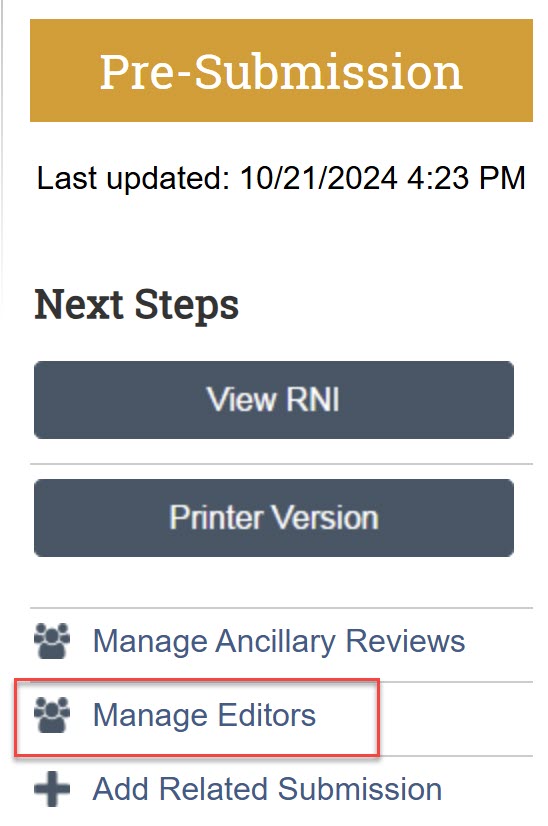
I need to edit an RNI that someone else on the study team submitted. How do I do this?
An RNI editor needs to give you edit access to the RNI. The person who created the RNI and the PI and any PI proxies of any related (linked) studies automatically have edit access. An RNI editor can also grant edit access to another member of the study team using the “Manage Editors” activity.
If an existing RNI Editor is unable to grant edit access, contact hsdreprt@uw.edu for assistance.
What’s the difference between a RNI and a study modification?
A report of new information (RNI) is used to report new information or events to HSD’s compliance office for review. For example, breaches of confidentiality, unanticipated problems, and DSMB reports should all be submitted as RNI. See SOP RNI Reporting by Researchers for more information. Study records cannot be updated with a RNI, so if a change to the study is needed, a study modification must also be submitted.
Technical Issues
I received an error after entering my UW NetID and password. What do I do?
Your UW NetID and password are used to access various programs/sites at the University. Try to access MyUW. If you are able to log in to MyUW, you may use the same ID and password to log into Zipline. Note that Zipline does not work with shared NetIDs.
If you cannot log into MyUW, close your browser and try again. If that does not work, please contact UW IT at help@uw.edu, or 206-221-5000.
If you can log into MyUW, but not Zipline, please contact hsdinfo@uw.edu.
I have a new UW NetID and now I can’t see my studies. Why not?
Zipline access is tied to your UW NetID. HSD cannot link your old and new UW NetIDs. To gain access to your studies, the PI or PI Proxy will need to add you to studies you need to access by submitting study team member modifications.
The consent form watermark is cut off when I print. What do I do?
The watermark located in the upper left hand corner will sometimes be cut off when printing out the finalized consent form. Select “Fit” or “Fit to Page” from the print menu before printing.
