After you submit an application to the NIH which uses an Adobe form set, you can have the system automatically update the submission status information. This information displays in both SPAERC and SAGE.
To request the status updates, your application must
- have a status of In OSP
- have a post-submission Grants.gov tracking number
To start the process:
- Open and unlock the application
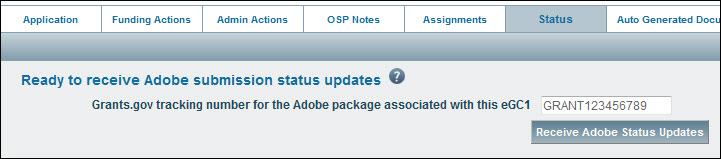
- Click on the Status tab
- Enter the complete tracking number (GRANT plus all digits) in the text box.
- Click the “Receive Adobe Status Updates” button to start the process.
Information about the submission status will display on the application’s Status page and the History & Comments page.
Status updates will begin to flow through to SPAERC within 10 minutes. SPAERC users can edit the Grants.Gov tracking ID while the application is still in “In OSP” status. Once you approve the application, you can no longer edit the tracking ID. If you need to change the tracking ID of an approved application, contact the SAGE Help Desk.
Receiving the status updates will also cause the Adobe Submission icon to appear on the tasklist for the application.
SAGE Suite-Wide
Session Timeout Handling Improvements
When any open SAGE Suite application is left idle for 40 minutes (this can vary if you have other applications open that require UW authentication), users will now see an on-screen warning that their SAGE session will time out in 5 minutes without further activity. Renewing your 45 minute session can be done by simply clicking “Keep Working” in the warning pop-up. If your session expires, the system will automatically redirect you to a login page. This change will provide clear and consistent handling of session time-outs, reducing user confusion and accidental data loss.
SAGE eGC1
Improved eGC1 Status Handling at the Time of Award
An eGC1’s status changes to “Awarded” when a child funding action is initially sent to GCA. However, there were certain circumstances when the eGC1 status wasn’t updating appropriately if the child funding action was returned to OSP and then re-parented to a new eGC1. To catch these cases, the eGC1 status will also update to “Awarded” if the child funding action is “Re-Sent by OSP” to GCA. Existing eGC1s in “Approved” status with a child funding action in “Processed” status were updated to “Awarded” status.
SPAERC
Update Subaward Expiration Rules (delayed release)
The nightly batch job that updates the subaward status was simplified, and now evaluates Subaward End Date only. If the Subaward End Date is in the past, then the status is changed to “Expired”. If a new modification is approved, the subaward status will be changed to Active once again.
New Non-Fiscal Compliance page
The eGC1 Compliance Questions and Explanations pages have been replaced by a new page called Non-Fiscal Compliance. This consolidation provides a more streamlined experience for preparers and reviewers.
Updated questions reflect current institutional needs, with improved clarity in mind. Links to supporting guidance have been added to assist preparers in understanding the policies and regulations behind each question. You can review the updated questions by visiting this page..

Design improvements make the page easier to navigate and gauge progress toward completion.

eGC1s that are in routing at the time of this release will display the old compliance questions that existed when the eGC1 was initially routed.
However, any eGC1 that is returned or withdrawn after the release will be reset with the new compliance questions. Answers to the previous questions will no longer display. Plan for additional time to re-complete that page in case a return or withdrawal is necessary.
Zipline and Hoverboard linked to eGC1
Human Subject (Zipline) and Animal Use (Hoverboard) protocols can now be linked to your eGC1. Searching by IRB Application Number or IACUC Protocol Number will allow you to pull in real-time information, reducing manual back-and-forth between systems and improving the accuracy of protocol details in SAGE.

Improved Collaboration
Send page link to PI, Reviewers
From any standard eGC1 page (excluding Grant Runner forms), the URL is now a direct link that you can copy and share with others who have access to that eGC1. This will be particularly useful when collaborating with the PI on completing the Non-Fiscal Compliance page.

Multi-User Editing (limited)
Currently, only one person can edit an eGC1 at a time. However, the Non-Fiscal Compliance page can now be edited, even while someone else is working in other areas of the eGC1. This will avoid situations where the PI could be blocked from contributing to the Non-Fiscal Compliance page while the administrator is currently editing other areas of the proposal.
SAGE Grant Runner
NIH Required Fields Added to HSCT
To assist researchers in filling out the Human Subjects & Clinical Trials (HSCT) form, NIH required fields are now indicated with an asterisk and red highlighting. These same fields will have validation messages when the user clicks “Show Form Errors” at the top of the form. All NIH errors and validations will continue to show when the full application is checked by clicking “Check for Errors” in the left navigation of SAGE.
eGC1 Data Sync Improvements with Grant Runner Forms
To help prevent data inconsistencies when multiple browser sessions are open, the data sync between the eGC1 and Grant Runner forms has been improved. When you make updates to information in the eGC1 that flows through to Grant Runner forms, those updates will now automatically sync with each save/refresh of the eGC1.
Improved Accessibility for Keyboard and Tab Order Functionality
To meet accessibility standards, all Grant Runner forms are now accessible using keyboard only functionality. In addition, tab order has been improved to be more logical and intuitive, reducing the number of times the user has to press the tab key.
Error Messages Enhanced
Question numbers have been added to error messages on all Grant Runner forms. This update will help users quickly identify the specific error within a form. This will be especially helpful for longer forms such as the new Human Subjects & Clinical Trials form.




