May 7, 2024
For the Record- May 7, 2024: Protecting Participant Data in Workday, WA State DCT Bill, More
In this Issue:
Protecting Research Participant Data in Workday
UW Finance Safeguards and Additional Measures For Researchers
UW Finance recently published information about how study participant payment data placed in Workday is protected from unauthorized access and disclosure. With the transition to Workday the process for paying research participants via check or Zelle was centralized under the Workday Miscellaneous Payment business process. UW Finance implemented the following safeguards to prevent potential violations:
- Limited Access: Access to research participant payment information is available through certain Workday reports. Only authorized personnel have access to reporting data in Workday. Access is restricted to individuals who need it to perform their job duties related to financial transacting on behalf of the UW.
- Data Storage/ Migration: Research participant payment information is not migrated to the University’s enterprise data warehouse (EDW). Participant names are contained solely in the Workday system.
- User Training: UW is working towards a central learning management tool. This will facilitate annual training on proper handling of sensitive data, security best practices, and procedures for reporting any potential breaches or security incidents.
- Audit Trails: The audit trail mechanisms within Workday track and monitor access to reports. This data allows UW to review and analyze user activity logs to detect any unauthorized access or suspicious behavior.
There are additional measures that researchers can take to ensure personal information in Workday is minimized, such as:
- Use Non-Identifying Titles for Grant Worktags: When creating the “short title” for research projects or grants in Workday, avoid using titles that that include a study acronym that is searchable on the web or has the condition under study (e.g., GR12346 HIV Study). This prevents the research participant name from being associated with any information that would reveal something personal about them (e.g., they have HIV because they are in an HIV study).
- Include Information in Consent Forms: If a particular study population may have concerns that their financial information is accessible to others outside the study team, you can provide additional disclosure information as part of the consent process. Sample language may be found in HSD’s Designing the Consent Process.
If you have any questions, contact UW Finance at https://finance.uw.edu/contact-us.
WA State Diversity in Clinical Trials Bill
Update
In our April 2, 2024 newsletter, we introduced the new WA State Diversity in Clinical Trials bill (2SHB 1745) and its requirements.
The UW intends to apply the bill requirements to all prospective UW research that meets the NIH definition of a clinical trial and where UW employees or agents are responsible for or engaged in recruitment and consent activities. This means that all new UW clinical trials submitted to the IRB on or after the UW policy effective date (TBD, summer 2025) should seek to improve enrollment of underrepresented groups applicable to their target study population. The target study population must be based on epidemiology/pathophysiology of the disease and/or the population that the intervention is intended to treat. Some exclusions will apply but must be justified by science, medicine, ethics, and safety. Phase 1, pilot, and feasibility studies, for example, may meet these criteria. This policy would:
- Apply regardless of where the interventions occur.
- Apply to studies relying on an external non-UW IRB for review.
- Be a condition of the UW agreeing to review on behalf of non-UW institutions and individuals.
The work towards implementation of the bill is under way and is being overseen by the UW Medicine Office of Healthcare Equity and led by a Strategic Leadership Committee comprised of interested parties and subject matter experts. There are also five working groups supporting various aspects of the bill: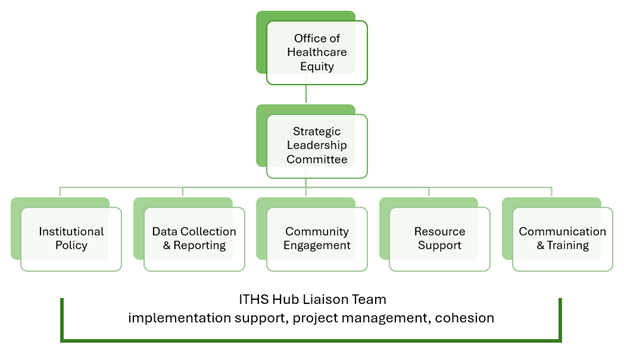
HSD is responsible for developing a set of institutional policies and supportive guidance for researchers to comply with the bill requirements. Other working groups are looking at such things as necessary pre- and post-award resources for researchers and how best to engage different community partners.
By the summer of 2024, the goal is to have:
- Institutional policies and guidance drafted
- Baseline and reporting metrics established
- Landscape review of available resources conducted
- Pre-award & post-award resource allocation pathways identified
- Phases of implementation determined (likely based on funding status)
Compliance with the new requirements would be one year from implementation of the new policies (i.e., summer 2025) though preparatory work at the pre-award/study design stage will need to occur before then. The new requirements would only apply to new studies submitted to the IRB on or after the 2025 implementation date.
There will be a series of town halls in the summer, dates TBD, where researchers and other interested parties will have an opportunity to ask questions and provide feedback. More information on these town halls and other details about the bill’s implementation will be provided in a future newsletter.
HSD Administrative Fee Increase for Industry Clinical Trials
Update
Effective July 1, 2024, HSD’s administrative fee will be increasing from $1933.70 to $2060.06. This one-time fee only applies to industry-sponsored-and-initiated clinical trials reviewed by a non-UW IRB where the contract was negotiated by UW’s Office of Sponsored Programs (OSP). This fee is charged because this type of research has an indirect cost rate that does not cover HSD’s costs for performing necessary administrative and regulatory oversight activities. The rate increase reflects updated costs for HSD to perform these activities. It is common for universities and research institutes to charge these fees for industry research.
Applicable external authorization requests submitted in Zipline on or after the effective date will have the new rate applied.
Contact hsdrely@uw.edu if you have any questions.
Advance Consultation about the UW as a Single IRB
Reminder
Serving as the Single IRB for a multi-site trial or multi-institutional collaborative research requires a significant commitment of limited HSD and UW IRB resources. Therefore, HSD must be consulted in advance of a funding application if a researcher wants to use the UW as the Single IRB for a study. HSD policy continues to be that it cannot guarantee UW IRB review of any multi-institutional research for which it has not been consulted in advance of any commitment to funding agencies or other institutions. Contact us at hsdrely@uw.edu if you would like to set up a consultation.